Abstract
Background
Primary central nervous system lymphoma (PCNSL) is a rare subtype of extranodal non-Hodgkin's lymphoma with poor prognosis. High-dose methotrexate (HD-MTX) is the cornerstone for treatment. However, there is no consensus on the best combination chemotherapy regimen. This study aimed to explore the efficacy and safety study of R2-MTX (Lenalidomide, Rituximab and Methotrexate) as a first-line induction regimen in the treatment of newly diagnosed PCNSLs.
Methods
This is a single-arm, prospective phase II study. Immunocompetent patients (18 to 75 years of age) with newly diagnosed PCNSLs were treated with 6 cycles of R2-MTX induction chemotherapy. Lenalidomide was dosed at 25mg daily (day 1-10); rituximab was dosed at 375mg/m2 (d0), MTX was dosed at 3.5g/m2 (d1). Every 21 days were as a course of treatment. Patients who did not reach CR after 6 courses of treatment were given salvage whole-brain radiotherapy (WBRT) to 30-36 Gy followed by a limited field to gross disease to 45 Gy. Then the patients received R2 maintenance (Rituximab 375mg/m 2 d1, lenalidomide 25mg d1-14, 29-42, 56 days per cycle) for 4 cycles. The primary endpoint is the objective response rate.
Results
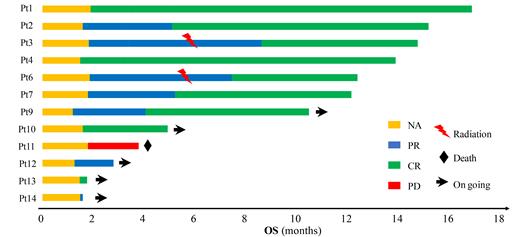
14 patients were enrolled from Feb 2020 to June 2021. Six (42.9%) patients were male and the median age was 59 (range 44-73). Ten (83.3%) patients were mediate or high-risk group (2-5 points) according to IELSG score. The last follow-up was July 15th 2021. Twelve patients were evaluable for response (one patient withdrew the consent; another patient was found with chronic lymphocytic leukemia). The overall response rate (ORR) was 91.7% (11/12). Nine patients achieved complete remission, and two patients achieved partial remission at the last follow-up. The median follow-up time was 11.3 (1.3-16.9) months. Only one patient did not respond and died for disease progression (Figure 1). The most common grade ≥3 adverse events (AEs) included elevated alanine transaminase (ALT) (16.7%), neutropenia (16.7%), thrombocytopenia (8.3%), infection (8.3%), and acute kidney injury (8.3%) (One patient with MTX overdose suffered from all these ≥3AEs). The most common grade 1-2 AEs were neutropenia (66.7%), anemia (58.3%), thrombocytopenia (16.7%) elevated ALT (41.7%).
Conclusion
R2-MTX regimen is an effective and well-tolerated combination treatment for newly diagnosed PCNSLs. More clinical data will be updated.
Keyword(s): Primary Central Nervous System Lymphoma, Lymphoma therapy, Methotrexate, Rituximab, Methotrexate, Lenalidomide
Trial registration: ClinicalTrials.gov NCT04934579.
Conflict of Interest: The authors declare that they have no conflict of interest.
Figure 1 Swimmer plots of all PCNSL patients evaluable for response.
Each bar represents one patient; the length of the bars shows the time between the initiation of treatment and the last follow-up.
No relevant conflicts of interest to declare.